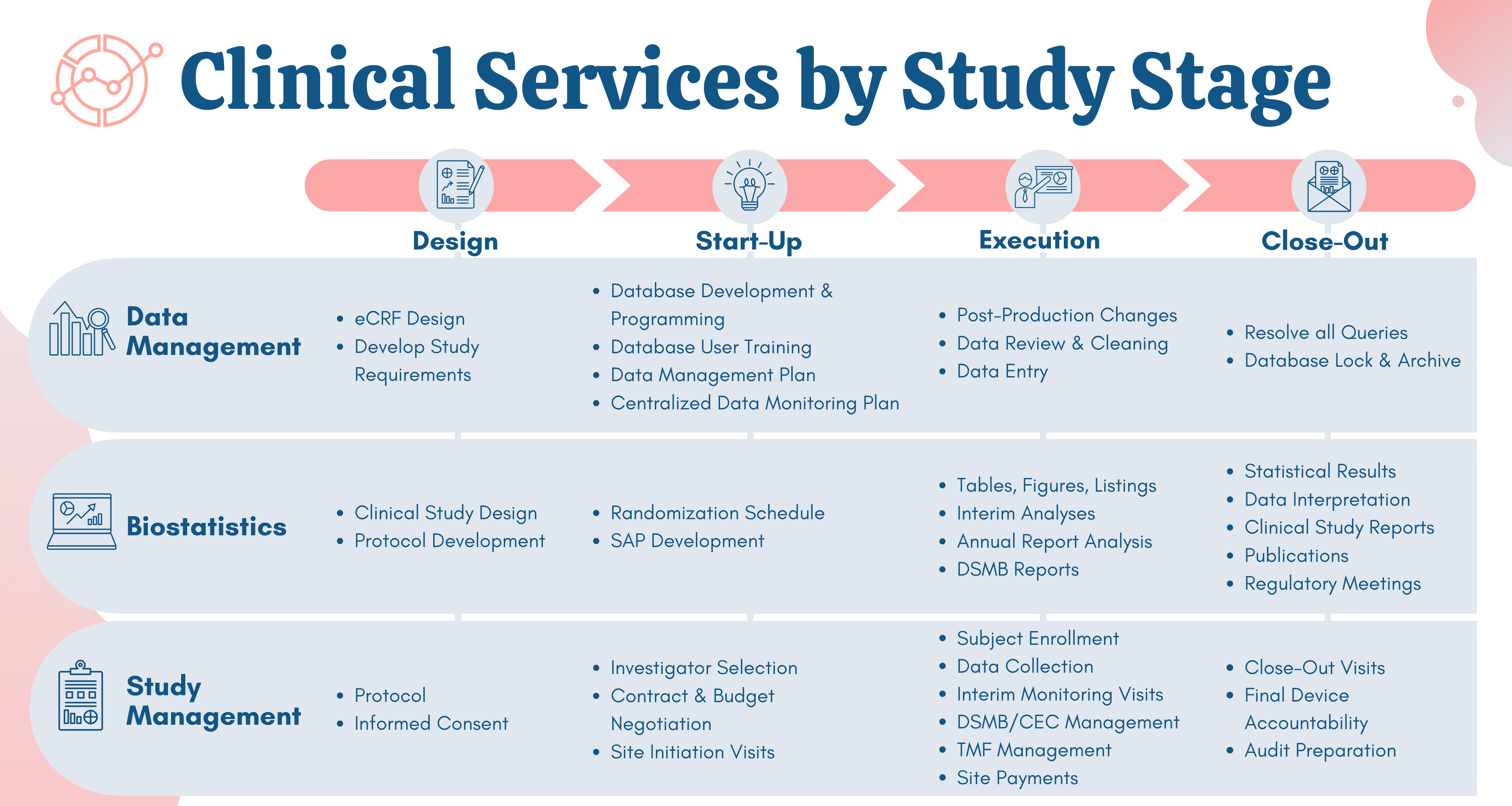
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups | The National Academies Press

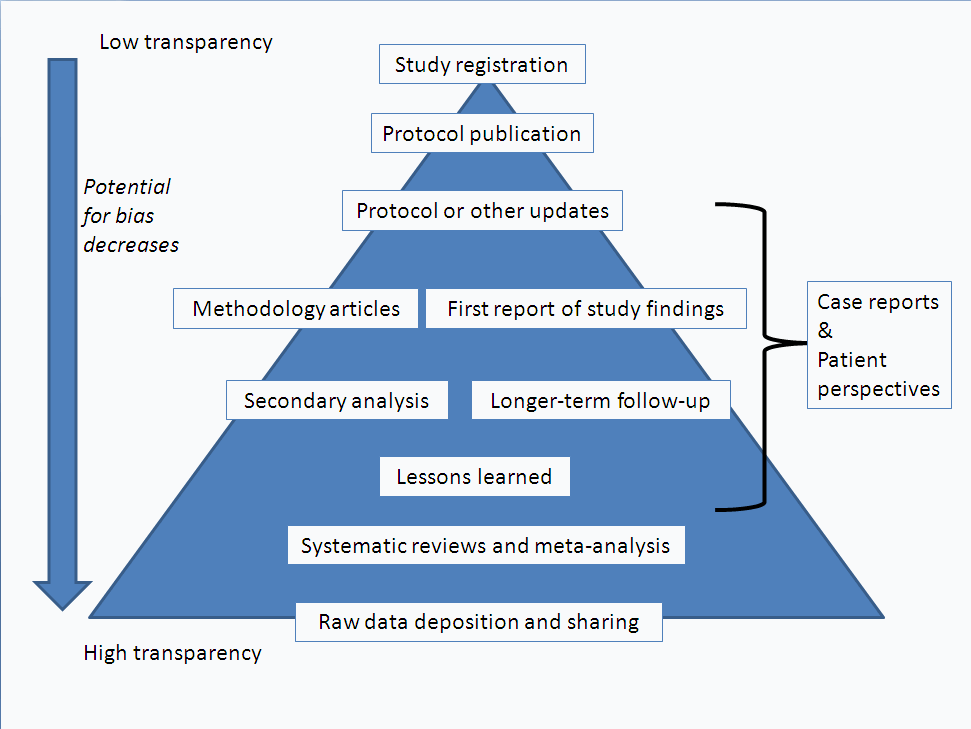
Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers | The BMJ
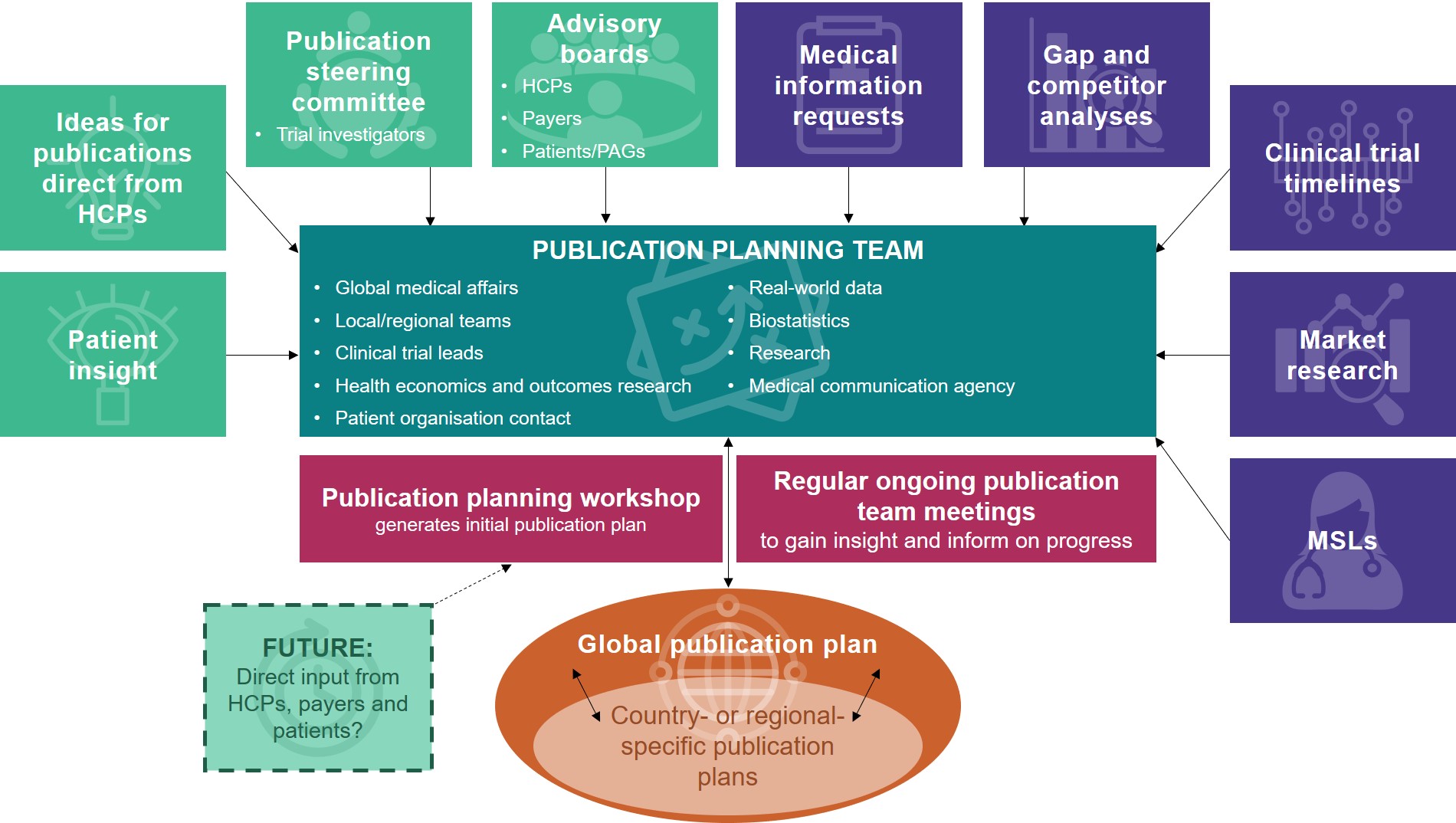
It Takes a Village: The Value of Publication Planning with Functional Partners and External Stakeholders | the Map
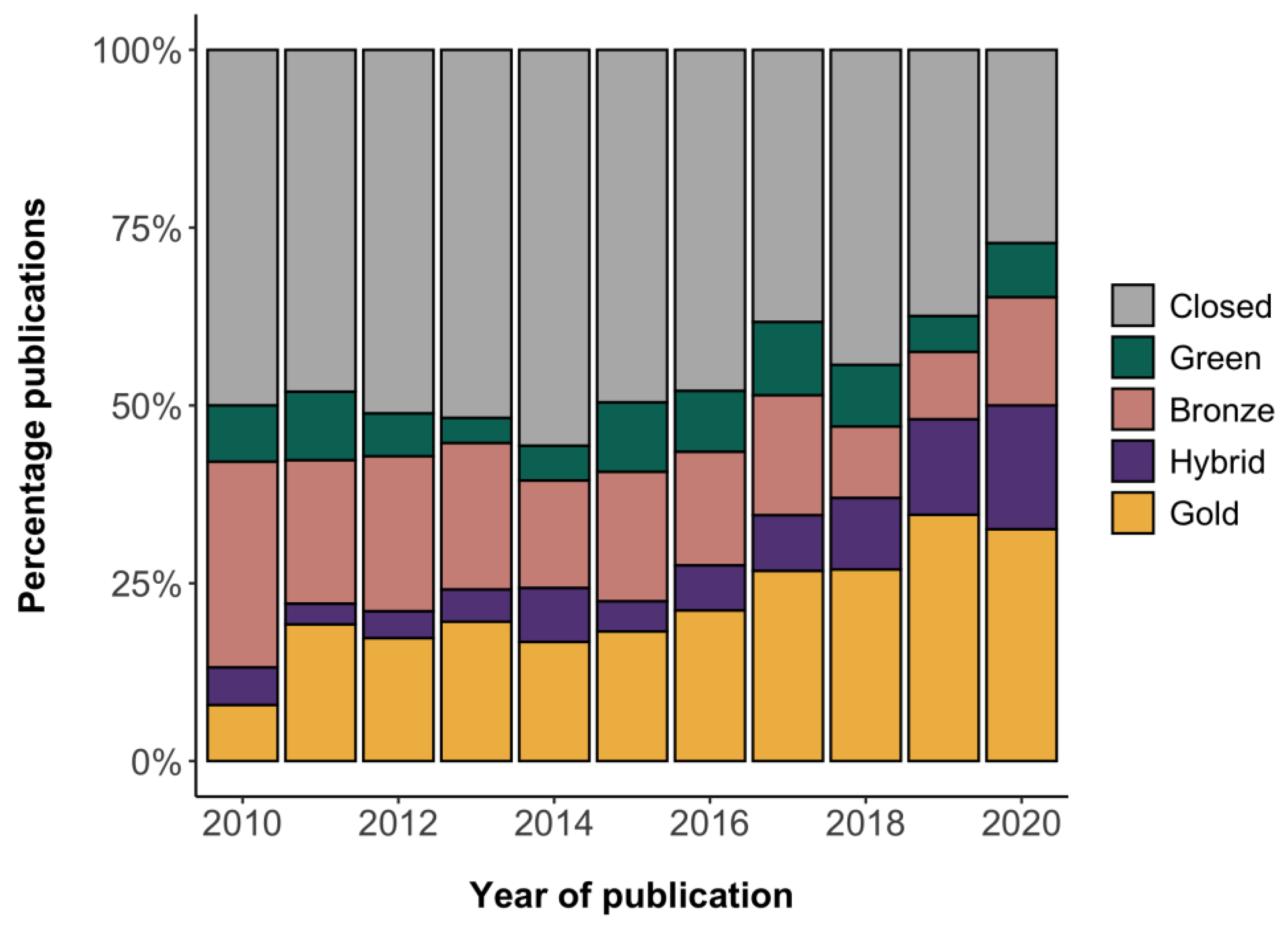
Publications | Free Full-Text | Leveraging Open Tools to Realize the Potential of Self-Archiving: A Cohort Study in Clinical Trials

Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning
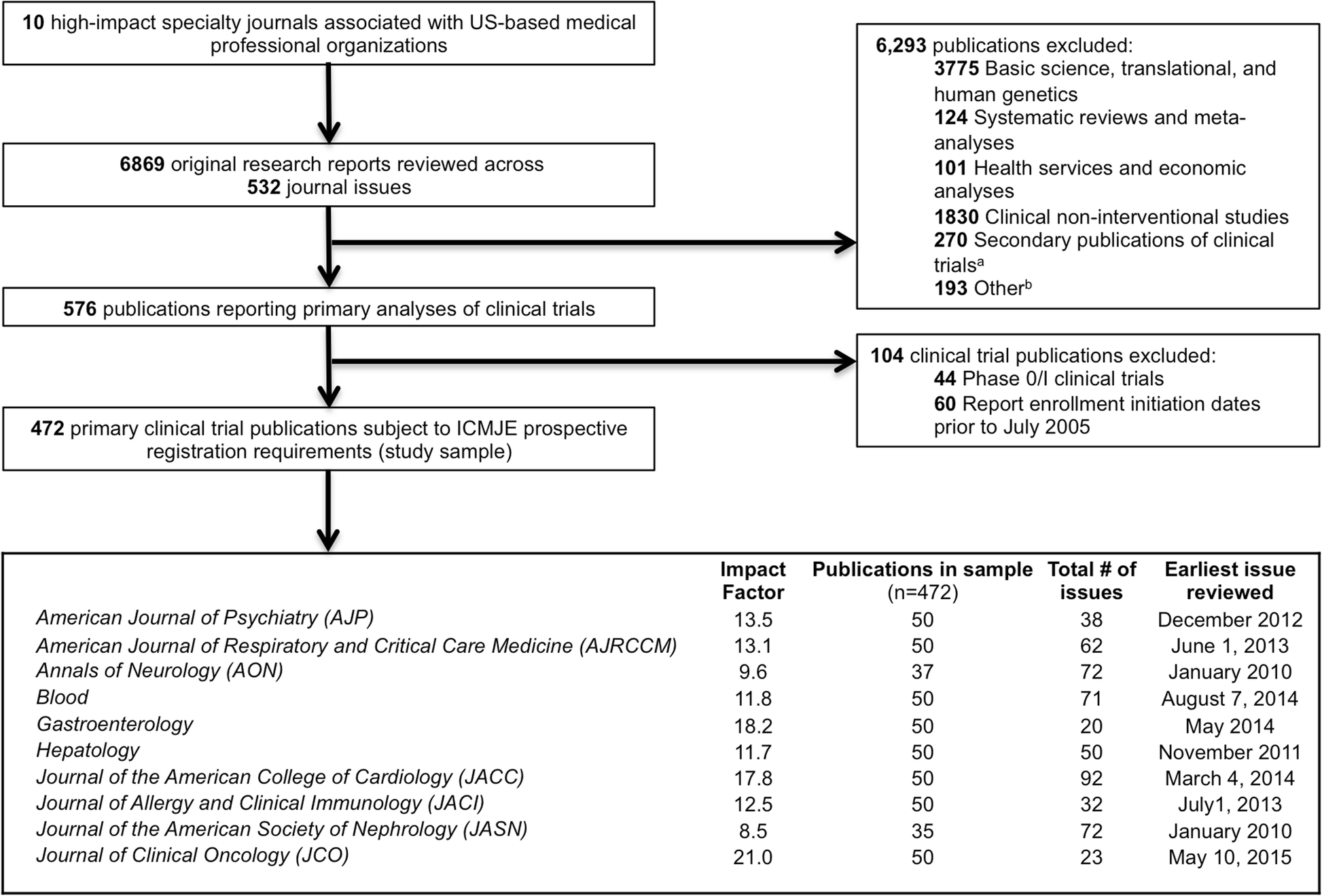
Adherence to the International Committee of Medical Journal Editors' (ICMJE) prospective registration policy and implications for outcome integrity: a cross-sectional analysis of trials published in high-impact specialty society journals | Trials
![PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/62bcc7885dac29517026f3ba577045d3b93dbb7b/2-Table2-1.png)